Abstract
Introduction: Undetectable minimal residual disease at <10-4 sensitivity (uMRD4 ) and intermediate MRD (<10-2 to ≥10-4) status in both peripheral blood (PB) and bone marrow (BM) are correlated with progression-free survival (PFS) and overall survival in patients treated with chemoimmunotherapy. However, the prognostic significance of uMRD4 in the setting of targeted agents has not been established, primarily due to the rare achievement of uMRD4 with B-cell receptor signaling pathway inhibitors (BCRi). Additionally, the multi-compartmental nature of CLL and differential effects of each therapy on these compartments could lead to differences in MRD level between PB and BM; therefore, relationship between PB and BM must be defined for each new treatment approach. Venetoclax is a targeted BCL-2 inhibitor approved for treatment of relapsed/refractory (R/R) CLL, and has achieved PB uMRD4 rates of 30% as monotherapy (Stilgenbauer, et al. J Clin Oncol. 2018; 36(19):1973-1980). In this post-hoc analysis we assess the concordance between MRD status in PB and BM and the prognostic significance of MRD levels achieved with venetoclax monotherapy.
Methods: MRD and PFS data from two phase 2 studies of venetoclax monotherapy in patients with R/R CLL were pooled: Study M14-032 (NCT02141282) of patients with R/R CLL who failed BCRi therapy and Study M13-982 (NCT01889186) of patients with R/R CLL (and 5 previously untreated) with del(17p). In both studies, venetoclax was administered at a final dose of 400mg/day after dose ramp-up until disease progression or prohibitive toxicity. Patients with informative PB or BM MRD data available were included in the analysis. MRD was evaluated by multi-color flow cytometry, with the majority of patients assessed at regional reference centers using assays based on the standardized method published by the European Research Initiative on CLL (ERIC) consortium (4-color in the EU; 6-color in Australia and North America). Quantitative MRD results were categorized as uMRD4, intermediate MRD, or high MRD (≥10-2). For the correlation analysis between PB and BM MRD, only patients with matched time points (same day) were included. The concordance of MRD between PB and BM was evaluated by linear correlation coefficient. PFS was evaluated using Kaplan-Meier estimates and hazard ratios between best PB MRD status, and estimated by the Cox proportional hazards regression.
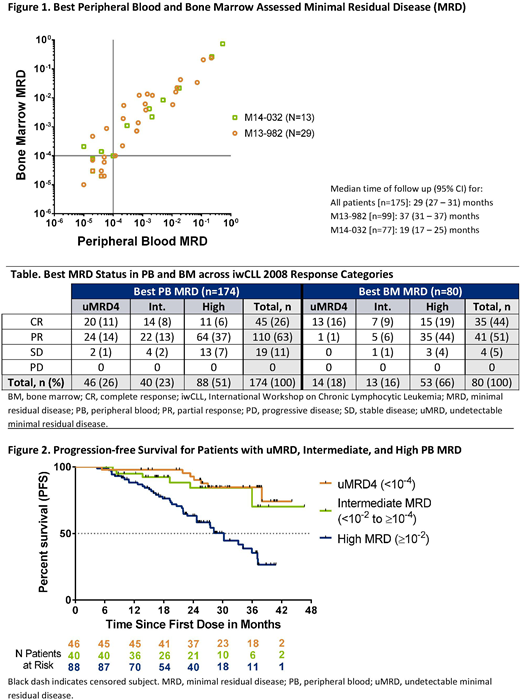
Results: Of the 285 patients from the two trials, 176 had informative MRD data in either PB or BM and were included in the analyses. For the PB and BM comparison, there were 42 samples with matched time points from 38 patients. PB MRD level correlated with BM MRD level (Figure 1; R2 = .96; P<0.001); no patients with uMRD4 in BM had contemporaneous detectable MRD in PB. Out of 174 and 80 patients with PB and BM MRD data respectively, uMRD4 and intermediate MRD were achieved across iwCLL 2008 response categories (Table; all CRs were confirmed in BM). Median times to attainment of PB uMRD4 (n=46) and PB intermediate MRD (n=40) were 14 (range: 3.5-37) and 8 (range: 3.5-28) months, respectively. Best PB MRD status was associated with PFS; outcomes were superior for patients who achieved uMRD4 (HR 0.20; 95% CI 0.09, 0.44; P<0.001) or intermediate MRD (HR 0.29; 95% CI 0.12, 0.68; P=0.005) vs high MRD. PFS rates at 24 months from commencement of therapy were 92.8%, 84.3%, and 63.2% for PB uMRD4, intermediate MRD, and high MRD patients, respectively (Figure 2).
Conclusions: Venetoclax monotherapy induced high rates of PB uMRD4 and intermediate MRD in both CR and PR iwCLL categories. PB and BM MRD levels achieved with venetoclax were highly concordant. Achievement of either uMRD4 or intermediate MRD in PB with venetoclax was associated with longer PFS.
Wierda:AbbVie, Inc: Research Funding; Genentech: Research Funding. Roberts:Walter and Eliza Hall: Employment, Patents & Royalties: Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone and royalty payments related to venetoclax; AbbVie: Research Funding; Genentech: Research Funding; Janssen: Research Funding. Ghia:AbbVie, Inc: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Sunesis: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Acerta: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding. Brown:Janssen: Consultancy; Abbvie: Consultancy; Acerta / Astra-Zeneca: Membership on an entity's Board of Directors or advisory committees; Sun Pharmaceutical Industries: Research Funding; Loxo: Consultancy; Beigene: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Morphosys: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy; Invectys: Membership on an entity's Board of Directors or advisory committees; Sunesis: Consultancy; Roche/Genentech: Consultancy; Pharmacyclics: Consultancy; Boehringer: Consultancy; Celgene: Consultancy; Verastem: Consultancy, Research Funding; Gilead: Consultancy, Research Funding. Stilgenbauer:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffmann La-Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmcyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cymbalista:Gilead: Honoraria; Janssen: Honoraria; AbbVie, Inc: Honoraria; Sunesis: Research Funding. Lamanna:Verastem: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jannsen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Seymour:Celgene: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding. Böttcher:Celgene: Research Funding; Genentech: Research Funding; AbbVie, INc: Honoraria, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria. Breuleux:Roche: Employment, Equity Ownership; Gilead: Equity Ownership; Basilea: Patents & Royalties; Novartis: Patents & Royalties. Chyla:AbbVie, Inc: Employment, Equity Ownership. Zhou:AbbVie, Inc: Employment, Equity Ownership. Nielsen:AbbVie, Inc: Employment, Equity Ownership. Kim:Abbvie: Employment, Equity Ownership. Potluri:AbbVie: Employment, Equity Ownership. Maher:AbbVie, Inc: Employment, Equity Ownership. Hillmen:Alexion Pharmaceuticals, Inc: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Research Funding; Novartis: Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences, Inc.: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.